Central Alabama Radiation Oncology is now certified to prescribe Optune Lua™ for the treatment of malignant pleural mesothelioma (MPM). This wearable and portable medical device is the first FDA-approved mesothelioma treatment in more than 15 years.


Optune Lua is approved for the first-line treatment of adult patients with unresectable, locally advanced or metastatic, MPM to be used concurrently with pemetrexed and platinum-based chemotherapy. In a clinical study, MPM patients treated with Optune Lua plus chemotherapy experienced a median overall survival of 18.2 months.
Optune Lua is a wearable and portable device that can be started through a virtual interaction. Optune Lua delivers Tumor Treating Fields to the region of the tumor. Tumor Treating Fields therapy, which uses electric fields tuned to a specific frequency, is designed to specifically target and directly disrupt dividing mesothelioma cells, inhibiting tumor growth and possibly causing affected cancer cells to die while sparing normal, healthy cells.
Optune Lua for MPM is classified as a Humanitarian Use Device (HUD), approved under the Humanitarian Device Exemption (HDE) which encourages companies to innovate in rare diseases with underserved patient populations. The FDA approved Optune®, another Tumor Treating Fields delivery system, under the Premarket Approval (PMA) pathway in 2011 for the treatment of recurrent glioblastoma (GBM) and in 2015 for the treatment of newly diagnosed GBM in combination with temozolomide. Since 2011, more than 15,000 patients with GBM have been treated with Tumor Treating Fields.
Of note, free transportation assistance, including travel, lodging and car rental may be available through CancerCare. For more information on applying, contact Charlotte Ference, LMSW at cference@cancercare.org or 800-813-4673.

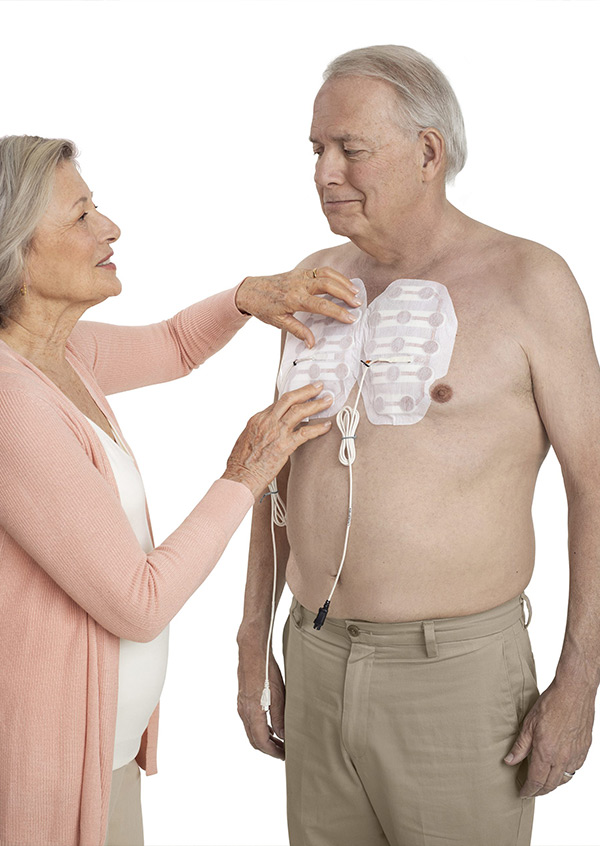
Indications for Use
Optune Lua is indicated for the treatment of adult patients with unresectable, locally advanced or metastatic, malignant pleural mesothelioma (MPM) to be used concurrently with pemetrexed and platinum-based chemotherapy.
Optune is intended as a treatment for adult patients (22 years of age or older) with histologically-confirmed glioblastoma multiforme (GBM).
Optune with temozolomide is indicated for the treatment of adult patients with newly diagnosed, supratentorial glioblastoma following maximal debulking surgery, and completion of radiation therapy together with concomitant standard of care chemotherapy.
Important Safety Information
Contraindications
Do not use Optune Lua in patients with MPM with implantable electronic medical devices such as pacemakers or implantable automatic defibrillators, etc. Do not use Optune in patients with GBM with an implanted medical device, a skull defect (such as, missing bone with no replacement), or bullet fragments. Use of Optune together with skull defects or bullet fragments has not been tested and may possibly lead to tissue damage or render Optune ineffective.
Use of Optune Lua for MPM or Optune for GBM together with implanted electronic devices has not been tested and may lead to malfunctioning of the implanted device.
Do not use Optune Lua for MPM or Optune for GBM in patients known to be sensitive to conductive hydrogels. Skin contact with the gel used with Optune Lua and Optune may commonly cause increased redness and itching, and may rarely lead to severe allergic reactions such as shock and respiratory failure.
Warnings and Precautions
Optune Lua and Optune can only be prescribed by a healthcare provider that has completed the required certification training provided by Novocure®.
The most common (≥10%) adverse events involving Optune Lua in combination with chemotherapy in patients with MPM were anemia, constipation, nausea, asthenia, chest pain, fatigue, medical device site reaction, pruritus, and cough.
Other potential adverse effects associated with the use of Optune Lua include: treatment related skin toxicity, allergic reaction to the plaster or to the gel, electrode overheating leading to pain and/or local skin burns, infections at sites of electrode contact with the skin, local warmth and tingling sensation beneath the electrodes, muscle twitching, medical device site reaction and skin breakdown/skin ulcer.
The most common (≥10%) adverse events involving Optune in combination with chemotherapy in patients with GBM were thrombocytopenia, nausea, constipation, vomiting, fatigue, medical device site reaction, headache, convulsions, and depression.
If the patient has an underlying serious skin condition on the treated area, evaluate whether this may prevent or temporarily interfere with Optune Lua and Optune treatment.
Do not prescribe Optune Lua or Optune for patients that are pregnant, you think might be pregnant or are trying to get pregnant, as the safety and effectiveness of Optune Lua and Optune in these populations have not been established.
Please click here to see the Optune Lua Instructions For Use (IFU) for complete information regarding the device’s indications, contraindications, warnings and precautions.
Please click here to see the Optune IFU for complete information regarding the device’s indications, contraindications, warnings and precautions.

